Hygienic design improves safety and reduces cost
Product safety and cost-effective production are key areas of focus for the pharmaceutical industry. Alfa Laval’s equipment is specially designed and built with hygiene in mind to address the areas of product safety and efficiency in pharmaceutical production.
DATE 2024-05-06 AUTHOR David WilesContamination of medicines by bacteria, cleaning chemicals or other products can lead to illness and even death amongst patients. Such avoidable tragedies can also lead to high compensation costs and reduced consumer confidence in the companies involved.
Ability to reduce cost without compromise
Against this background, pharmaceutical companies are under pressure to reduce their use (and thereby cost) of raw materials, energy and water. There is also a drive to get the most out of the raw materials. Cleaning in particular is a major expense for these companies, consuming water, electrical energy and time.
Many of our end users are beginning to understand that it is the small details in hygienic design that are important. It is not just the equipment itself but its cleanability, the quality of raw materials, its surfaces, how it is manufactured and how it matches the other stages of the production process that affect product safety. This applies not only to the pharmaceutical industry but also to the food industry.”
Per-Åke Olsson, Pharma Industry Manager at Alfa Laval
Select equipment designed for ease of cleaning
Centrifugal pumps, for example, are potential sources of contamination because of the presence of small crevices where bacteria or contaminants from previous manufacturing cycles can accumulate.
“Normally you clean your system in place without disassembling it,” says Olsson. “The cleaning liquid circulated in the system cannot always reach to the bottom of a crevice to clean it. When you introduce your next medicine, the residue left in the crevice will contaminate it.”
Industry regulations demand that equipment be designed so that it can be easily and thoroughly cleaned. Improved product safety through hygienic design will also reduce production costs. “A component that is difficult to clean requires more cleaning liquid and water as well as more time, and so it costs more,” says Olsson.
Equipment design: Eliminate conditions for bacterial growth
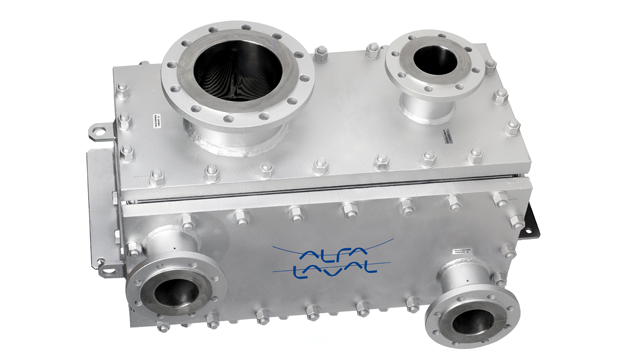
One example of a hygienically designed plate heat exchanger is Alfa Laval’s Compabloc Free Flow, designed for solvent condensing in the API industry. It is crevice-free, fully drainable and without contact points on the product wetted side. The design ensures efficient cleaning and minimizes the risk of contamination between batches.
Alfa Laval’s unique point-of-use cooler, Pharma-X, specially designed for pharmaceutical water systems also boasts a number of features that eliminate the risk of contamination, such as the sub-loop design, where hot water flows continuously in the heat exchanger, when on standby, and because of this the water temperature is always more than 65˚C. This means the typical bacteria found in pharmaceutical water systems cannot grow.
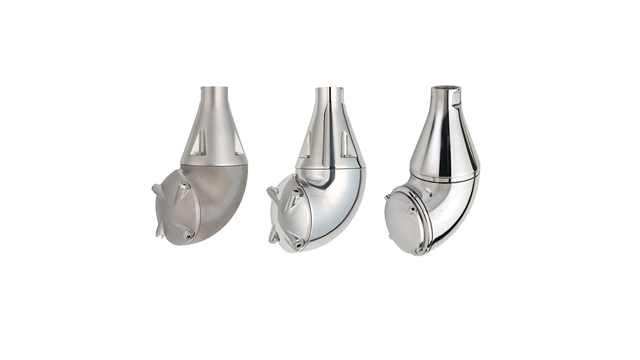
Another unique product that helps minimize product contamination is the LeviMag UltraPure magnetic mixer. As opposed to traditional magnetic mixers, the levitated design with floating impeller means there is no contact with the male bearing.
“The traditional design makes it difficult to drain and clean. It also generates wear particles,” says Olsson. “The levitated design solves this problem with a magnetic coupling that lifts the impeller from the surfaces of the bearing to make it easier to clean and drain.”
The design also minimizes wear particles and enables the mixer to operate at a very low velocity. The resultant gentle treatment of the product improves yield from the raw material.
Savings on water and energy costs
Alfa Laval’s tank-cleaning products contribute to improved hygiene in the pharmaceutical industries while having a considerable impact on water and energy costs. By using SaniJet rotary jet heads, one drug manufacturer reduced cleaning time by 70%, while using just a tenth of the water and heating energy compared with its previous solution which used static spray balls. “These nozzles give a very high impact on the surface and operate in a three-dimensional and very precise pattern,” says Olsson. “So we are both increasing safety through better coverage and cleaning of the tank and reducing consumption of cleaning liquids and water.”
Quality assured tubes and fittings
The tubes and fittings that link the various components together are often neglected by the pharmaceutical industry, but these can be a major source of contamination. In many systems, these parts can account for some 90% of the total product wetted surface.
“A tube is not just a tube or a piece of metal with a hole through it,” says Olsson. “What is important is how the tubes are polished and cleaned, how they are manufactured and the quality procedures that secure high-quality raw materials and manufacturing procedures.”
Alfa Laval’s tubes and fittings are backed up by a rigorous quality system with 100% inspection and well-proven and validated standard operating procedures.
Equipment designed for new regulations
Strict regulations have made the pharmaceutical industry somewhat slow in moving over to new technologies. The European pharmaceutical industry is at the forefront of adopting new technologies for improved product safety and cost-efficiency, such as improved hygienic design and continuous manufacturing instead of batch manufacturing. Also, the pharmaceutical authorities are now trying to change the conservatism in the industry in order to improve both safety and efficiency. New regulatory initiatives include the US Food and Drug Administration’s “PAT [Process Analytical Technology] – A Framework for Innovative Pharmaceutical Development, Manufacturing and Quality Assurance” and ICH Q8-Q11, which covers a risk-based approach to development and manufacturing of drugs.
Surveys show that companies are not expecting revolutionary change in the kind of equipment they use in the near future, but rather small incremental improvements. These improvements will probably be seen in equipment designs that facilitate the new regulatory initiatives, which contribute to improved efficiency, safety and product quality. By adopting a risk-based approach on equipment design to prevent contamination, both equipment supplier and the pharmaceutical industry will focus more on hygienic equipment designs.
Correct documentation facilitates risk-based analysis
Another area that will come more into focus is documentation of equipment. Correct documentation is essential to carry out an accurate risk-based analysis on contamination risks from equipment.
“We have a very detailed and thorough documentation on our products, called Alfa Laval Q-doc,” says Olsson. “We explain, for example, which materials we use, how we manufacture our components and how we control the quality of incoming and outgoing goods. By having this information it is possible to evaluate if there are risks for contamination.”
Contamination could come from the material itself or from additives that have been used during manufacturing. “The documentation certifies that the equipment has been fully controlled and manufactured in accordance with validated standard operating procedures,” says Olsson. “This gives full transparency and long-term peace of mind and further contributes to product safety.”